The Mysterious Allotropes of Carbon |
|
|
![]()
There is rather a mystery surrounding the Question: How many crystallographic forms, or allotropes, of Carbon exist? The information below is a sampling of opinions. We invite your vote on the topic.
![]()
| |
(CNT) is the material lying between fullerenes and graphite as a new member of carbon allotropes. The study of CNT has gradually become more and more independent from that of fullerenes. As a novel carbon material, CNTs will be far more useful and important than fullerenes from a practical point of view, in that they will be directly related to an ample field of ``nanotechnology''. |
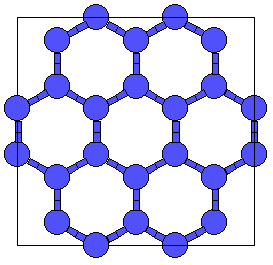 |
This is related to the hcp (A3) lattice in the same way that diamond (A4) is related to the fcc lattice (A1). It can also be obtained from Wurtzite (B4) by replacing both the Zn and S atoms by Carbon. |
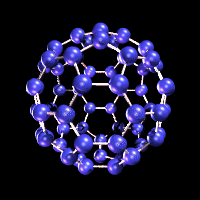 Fullerenes
are the third stable allotrope of carbon (besides graphite and diamond) and
were first discovered in molecular beam experiments in 1985. This won the team
of scientists involved the 1996
Nobel prize in chemistry for the discovery of
C60 . (Harold
W. Kroto,
Robert F. Curl
and Richard E. Smalley.
Fullerenes
are the third stable allotrope of carbon (besides graphite and diamond) and
were first discovered in molecular beam experiments in 1985. This won the team
of scientists involved the 1996
Nobel prize in chemistry for the discovery of
C60 . (Harold
W. Kroto,
Robert F. Curl
and Richard E. Smalley.
As can be seen from the graphic , fullerenes such as C60 are closed, highly symmetric molecules...
See also Fullerene und Fulleride Structures
Graphite
 and diamond
and diamond
 are two
of the most interesting minerals. They are identical chemically - both are
composed of carbon (C), but physically, they are very different. Minerals
which have the same chemistry but different crystal structures are called
polymorphs
are two
of the most interesting minerals. They are identical chemically - both are
composed of carbon (C), but physically, they are very different. Minerals
which have the same chemistry but different crystal structures are called
polymorphs
 tend to be rather yellow. Synthetic diamonds are the hardest material with
good physical and chemical properties of very high compressive and corrosion
resistance. They are widely used in machine, metallurgy, oil, geology,
building materials, electron, instrument and meter, national defense industry
and frontiers of technology.
tend to be rather yellow. Synthetic diamonds are the hardest material with
good physical and chemical properties of very high compressive and corrosion
resistance. They are widely used in machine, metallurgy, oil, geology,
building materials, electron, instrument and meter, national defense industry
and frontiers of technology.A young man buys a beautiful diamond engagement ring to give to his fiancé. A high-school student takes a chemistry test using a graphite pencil. A woman falls asleep in her car with the engine running and dies from carbon monoxide poisoning. Do these three seemingly unrelated situations have anything in common? Yes, they absolutely do! .... (See here for the whole essay!)
I vote for many more than 4 allotropes, but the form I am mainly interested in is the C84
crystal which, I believe, was the upshot of an accidental discovery at (?) Arizona State in about (?) 1985.
This incident was related in Jim Baggot's book "Perfect Symmetry: The Accidental Discovery of Buckminsterfullerene" (Oxford University Press, 1994)
On page 153 there are three photomicrographs, at three different magnifications, showing the result of mixing benzene with C60-rich soot and applying heat. The two upper ones are much alike; maximum magnification, say, 1500X. the third one is electron microscope picture, and appears to be about ten times as big, or 15,000X ... don't be fooled by the indicated scale, which suggests that 1mm ~ 1nm (nanometer), which would make the crystal as it appears, about 2 nm thick. But the crystal is obviously very dense, and as the diameter of the C60 itself is about One nm, the scale in the picture must be off. My thesis is regarding the interaction between the C60 and the C6H6. It is possible to select four sites, tetrahedrally symmetrical, on the C60. Each site is a hexagon, surrounded by three hexagons and three pentagons alternating. At the central hexagon, unhook all six carbons and insert six additional. This will make an outward-pointing opening surrounded by three hexagons and three heptagons, alternating. Repeat the process for the other three sites and you have the C84 molecule. These interconnect to form the crystal best shown in the lower photo on page 153.
Reaction? §C60 + 4§(C6H6) + heat > §C84 + 12§H2 maybe.
One more thing: the C84 molecule as described above is chiral, and each molecule must be connected to one of opposite "twist."
I have tried several times to push this idea and have never had any response. I don't know if already old news, or if there are patent rights involved or what. Anyway, if this interests you I will send you a stereogram of the C84 crystal: just give me a postal address.
This web page is a great idea. I thought I was the only
one who was confused. 12-15 years ago I remember hearing something about a
"fourth carbon" called "carbyne". I wish I could remember were I saw that.
In between the perfect forms you have listed are a large variety of carbon structures each having distinct physical properties different from graphite, amorphous carbon, diamond, fullerines, and lonsdaleite. I call these allotropic forms because their higher order structure has not adequately been described by simple combinations of the above structures. If these are not allotropic forms what are they and what should we call them?
First consider the variation of Chemical Vapor Deposited (CVD) Graphite which has a turbostratic form (hexagonal with kinks) that varies in density and degree of order with deposition temperature (900 - 2000 C). One form of CVD carbon rings like a bell is very hard and is bright silver in color. Other different and unusual "phases" can be obtained by CVD deposition onto diamond powder.
Next consider the large variety of structures found in carbon fibers. Nobody said allotropic forms must be natural. These structures depend on the precursor and processing. Certainly these complex 3 dimensional structures do not fit into any of your listed forms. The most commons precursors are Rayon, PAN and Pitch. All yield different fibers, with various structures, densities and mechanical properties. It is interesting to decide how to classify higher order structures not based on crystallographic unit cells or total randomness.
Finally somewhere in between glassy carbon and fullerines is the remainder of the class of structures based on soot and carbon blacks. These materials have unique properties. Because of there individual and agglomerated structure of the soots and carbon blacks they have properties vastly different than glassy carbon.
According to my dictionary, which is not very technical, an allotrope has different properties with the same composition. Even in the world of polycrystalline graphite, different grades have different properties. Actually, I really feel there are only two allotropes; SP2 bonded (hexagonal) and SP3 bonded (tetragonal).
It also seems that you should make a distinction between HOPG (highly ordered pyrolytic graphite or single crystal graphite) and polycrystalline synthetic graphite which is what most people call 'graphite'. There are also fibers, where the crystallographic planes are tubular and layered like an onion. Although these have different properties, I do not consider them allotropes.
About 15 years ago, I read the LBNL produced a true 'white' graphite that had unique properties, but I was never able to get more information on it. Also be aware that many people refer to boron nitride as 'white graphite'.
After I E-mailed my vote, I decided you might want this excerpt from a paper I did for a Semiconductor meeting. I converted it to rich text format, so it may be a little scrambled.
![]()
 (Amorphous, the very hard
black stuff, not soot) Unlike graphite, glassy carbon has no long range
order in three dimensions. This produces a material which is chemically more
inert than graphite. Low CTE and thermal conductivity make glassy carbon
powder useful as a filler in metals, ceramics and plastics
(Amorphous, the very hard
black stuff, not soot) Unlike graphite, glassy carbon has no long range
order in three dimensions. This produces a material which is chemically more
inert than graphite. Low CTE and thermal conductivity make glassy carbon
powder useful as a filler in metals, ceramics and plasticsBut perhaps some of these are the same as others, or imaginary? In our democratic attempt to find the truth, we invite you to vote on where the truth lies: for 2 Allotropes or for 3 Allotropes or for 4 Allotropes or for More Than 4 Allotropes. Please indicate which they are!
![]()
Images and webpage designs © 2001-2026 your webmaster, jb and Dendritics Inc. [-]