Kestrel on Allotropes |
|
|
![]()
Kestrel Michaud's April 2004 High School essay on Carbon Allotropes
INTRODUCTION
A young man buys a beautiful diamond engagement ring to give to his fiancé. A high-school student takes a chemistry test using a graphite pencil. A woman falls asleep in her car with the engine running and dies from carbon monoxide poisoning. Do these three seemingly unrelated situations have anything in common? Yes, they absolutely do!
The diamond in the ring, the graphite in the
pencil, and the fullerene carbon monoxide are all allotropes of carbon.
Allotropes of carbon are so important to us that if they were to disappear off
the face of the earth right now, all life would cease to exist. Carbon is the
basis of life. Without allotropes, carbon could not exist; without carbon, life
itself would not be possible.
What is an allotrope?
By definition, allotropy is “the property that certain chemical elements and compounds have of existing in two or more different forms, as carbon in the form of charcoal, diamond…” (Webster’s New World College Dictionary: Fourth Edition, 2000) An allotrope is a form of an element that exhibits allotropy (Webster’s New World College Dictionary: Fourth Edition, 2000). Some elements, such as carbon, can exist in different forms, ie., a group of carbon atoms can bond to each other in different structural formations using double and triple bonds. For instance, the bonds between the carbon atoms in a molecule of buckminsterfullerene form pentagons and hexagons that fit together in the shape of a soccer ball.
Carbon has three different allotropes: diamonds, graphite, and fullerenes (Tim Appenzeller, 2004). Allotropes form through different manners and have different properties from each other (Wikipedia, 2004.).
Allotropes of an element are entirely
different from the phases of an element. The four possible phases of elements
are solid, liquid, gaseous, and plasma. In different phases of an element, the
bonds formed between the atoms are physically different. “Allotropes of any
element can be in any state, gaseous, liquid, or solid” (Wikipedia, 2004).
(Wikipedia, 2004). In an allotrope, the bonds between the similar atoms are
chemically different than the bonds in the original element.
Why does carbon form allotropes?
Carbon is different from any other element. It
has a unique characteristic that it is capable of bonding to itself and other
elements through the use of single, double, and triple bonds. No other element
can do this as readily as carbon. This trait causes carbon to easily “bind to
itself to form chains and rings” (Davis, Raymond E., PH.D., Metcalfe, H. Clark,
Williams, John E., Castka, Joseph F., 2002) and “to bind [with] other elements
in different arrangements” (Davis, Raymond E., PH.D., Metcalfe, H. Clark,
Williams, John E., Castka, Joseph F., 2002). When carbon binds to itself in
chains and rings it forms allotropes.
DIAMONDS
What is a diamond?
Most people probably think of carbon as a black chalky substance, such as coal. These same people would probably be very surprised to find that a diamond is actually 100% carbon, whereas coal is only 92% carbon ( Tim Appenzeller, 2004).
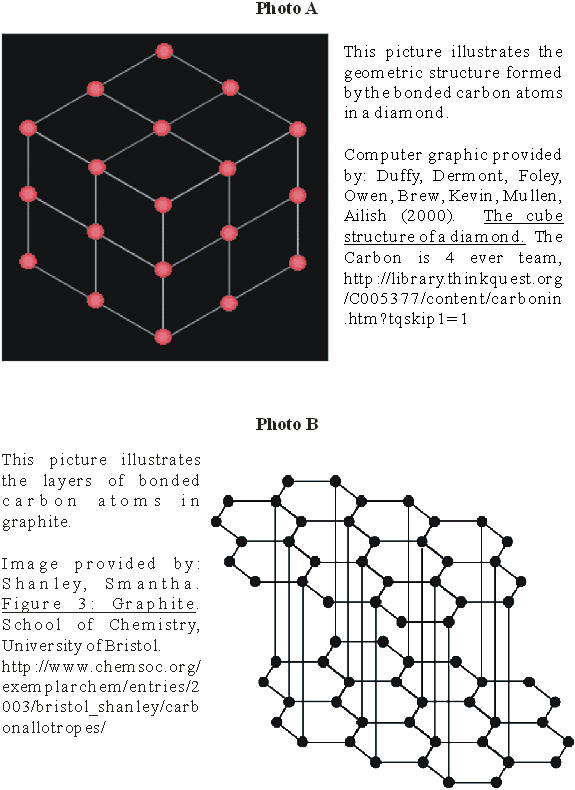
A diamond is the hardest naturally-occurring
substance known to mankind. Diamonds are generally clear or translucent and are
also an excellent insulator of electricity. They are the only precious gems that
are composed of a single element: carbon. These carbon atoms are crystallized in
a three-dimensional geometric pattern which gives the diamond its cubic shape.
(See Appendix A, photo A.) (Wikipedia, 2004.)
How do diamonds form?
The formation of diamonds occurs between 120
and 200 kilometers underground. Rocks in the upper mantle of the Earth melt. The
carbon from these rocks is forced lower into the earth where it finally
condenses into new rock. If the temperature and pressure is correct, the carbon
will form into diamonds. The diamonds then eventually rise to the surface. (Ravi
Kewalramani).
How do we use diamonds in society today?
Only a quarter of the diamonds mined are then
cut into jewelry, (Ravi Kewalramani). The vast majority of mined diamonds are
used in industry. Since diamonds are one of the hardest substances known to man,
they can cut through pretty much everything. For example, diamond-edged saws are
commonly used by masons to cut stone.
GRAPHITE
What is graphite?
Graphite is opaque silver-black in color and is one of the softest minerals known to man. “Graphite is [also] the most stable of the [carbon] allotropes.” (Samantha Shanley, School of Chemistry, University of Bristol.) The structural formation of graphite causes it (graphite) to be a good conductor of electricity.
In graphite, the carbon atoms bond together in
layers, with each layer being parallel to the next and previous one (Samantha
Shanley). These layers allow graphite to flake apart. When you write with a
pencil, the layers flake off of the graphite in the pencil and onto the page.
(See Appendix A, photo B.)
How do we use graphite in society today?
Graphite is used on the two electrodes of an
arc lamp. (“The arc lamp produces light by the sparking…of a high current
between two carbon rod electrodes” (Wikipedia, 2004).) Graphite can be found in
wooden pencils and in thin sticks which are used as refills for mechanical
pencils. A third place that carbon is used in society is as a dry lubricant for
machines in industry. (Wikipedia, 2004)
FULLERENES
What is a fullerene?
In the mid-1980s, Richard E. Smalley, Robert
F. curl, and Harold W. Kroto discovered the new form of compounds known as the
fullerene (Davis, Raymond E., PH.D., Metcalfe, H. Clark, Williams, John E.,
Castka, Joseph F., 2002). “The 1996 Nobel Prize in chemistry was awarded to
[Smalley, Curl, and Kroto]…for their discovery of fullerenes.” (Davis, Raymond
E., PH.D., Metcalfe, H. Clark, Williams, John E., Castka, Joseph F., 2002). M
Fullerene is short for Buckminsterfullerene (C60). This allotrope of carbon was
named for Richard Buckminster Fuller, who, among other things, invented the
geodesic dome. (Siqueira, Rodrigo A.). (See Appendix B, Photo A.). Because the
geodesic dome is similar to chemical bond structure of the fullerene, the name
was thought appropriate. A fullerene (C60, C70, C80, C90, etc. (Wikipedia,
2004)) is formed when “carbon containing materials are burned with limited
oxygen” (Davis, Raymond E., PH.D., Metcalfe, H. Clark, Williams, John E.,
Castka, Joseph F., 2002). Fullerenes can take different forms such as a sphere,
tube, ellipsoid, or ring (Wikipedia, 2004). The fullerene sphere structure has
also been nicknamed the Buckyball due to its resemblance to a soccer ball
(Davis, Raymond E., PH.D., Metcalfe, H. Clark, Williams, John E., Castka, Joseph
F., 2002). The fullerene tube structure is often referred to as a nanotube
(Wikipedia, 2004).
What are some properties of the fullerenes?
Since the dozens of carbon bonds in the
fullerene structures are incredibly stable, fullerenes have a very low
reactivity. This trait also causes fullerenes to be “insoluble in many solvents”
(Wikipedia, 2004). (Wikipedia, 2004).
An unusual characteristic of fullerenes is that they can trap other atoms inside
their structures. Evidence of an impact from a meteor “at the end of the Permian
period was found by analyzing Noble Gases” (Wikipedia, 2004) preserved in this
manner. (Wikipedia, 2004).
Another property of fullerenes that has only
recently begun to be tested is superconductivity (Wikipedia, 2004).
“Superconductivity is a phenomenon occurring in certain materials at low
temperatures, characterized by the complete absence of electrical resistance and
the damping of the interior magnetic field (the Meissner effect)” (Wikipedia,
2004).
Where can fullerenes be found and do we use them in society today?
Fullerenes have been discovered to exist in various geological materials in the soot produced when a candle burns (Samantha Shanley).
“Scientists are currently trying to find
practical uses for these substances” (Davis, Raymond E., PH.D., Metcalfe, H.
Clark, Williams, John E., Castka, Joseph F., 2002), although “In April 2003,
fullerenes were under study for potential medicinal use--binding specific
antibiotics to the structure to target resistant bacteria and even target
certain cancer cells such as melanoma” (Wikipedia, 2004).
CONCLUSION
Diamond-edged saws cut stone for masons. Graphite electrodes allow electricity to jump between them, creating light (Wikipedia, 2004). Fullerene molecules preserved Noble Gas molecules; these Noble Gas molecules then give testimony to a meteor impact that occurred in millennia past (Wikipedia, 2004). Not only do the allotropes of carbon provide practical uses, these unobtrusive structures are essential to our existence. Carbon is the basis for life on Earth and is a part of every living thing. Without allotropes, carbon could not exist; without carbon, life itself could not exist
BIBLIOGRAPHY
Agnes, Michael, Editor in Chief, Guralnik,
David B., Editor in Chief Emeritus (2000). Webster’s new world college
dictionary: Fourth edition. Foster City, California: IDG Books Worldwide, Inc.
Davis, Raymond E., PH.D., Metcalfe, H. Clark, Williams, John E., Castka, Joseph
F. (2002). Modern Chemistry. Austin, Texas: Holt, Rinehart, and Winston.
Kewalramani, Ravi. Diamond formation: How and where diamonds are formed. Gem
Sutra,
http://www.gemsutra.com/diamonds.html
Kewalramani, Ravi. Diamond formation: Diamond’s journey to the surface?. Gem
sutra,
http://www.gemsutra.com/diamonds.html
Borchard, John (2002). The mysterious allotropes of carbon. Dendritics Gemscale
Museum,
http://www.dendritics.com/scales/c-allotropes.asp
(2003). The hexagonal graphite (A9) crystal structure. Naval Research Laboratory
Center for Computational Materials Science,
http://cst-www.nrl.navy.mil/lattice/struk/a9.html
(2003, 2004). Wikipedia: The free encyclopedia. Media Wiki, pages
http://en.wikipedia.org/wiki/Allotrope ,
http://en.wikipedia.org/wiki/Graphite ,
http://en.wikipedia.org/wiki/Arc_lamp ,
http://en.wikipedia.org/wiki/Lubricant ,
http://en.wikipedia.org/wiki/Superconductivity ,
http://en.wikipedia.org/wiki/Fullerenes ,
http://en.wikipedia.org/wiki/Allotrope
Siqueira, Rodrigo A., A short page about Buckminster Fuller (Bucky).
http://www.lsi.usp.br/usp/rod/bucky/buckminster_fuller.html
Shanley, Smantha. Carbon allotropes .com: Introduction to carbon allotropes.
School of Chemistry, University of Bristol.
http://www.chemsoc.org/exemplarchem/entries/2003/bristol_shanley/carbonallotropes
Appenzeller, Tim (2004). The case of the missing carbon. National Geographic,
Vol. 205, No. 2, 88-117.
![]()
Images and webpage designs © 2001-2026 your webmaster, jb and Dendritics Inc. [-]